Section Branding
Header Content
What causes Alzheimer's? Study puts leading theory to 'ultimate test'
Primary Content
An idea that has propelled Alzheimer's research for more than 30 years is approaching its day of reckoning.
Scientists are launching a study designed to make or break the hypothesis that Alzheimer's is caused by a sticky substance called beta-amyloid. The study will give an experimental anti-amyloid drug to people as young as 18 who have gene mutations that often cause Alzheimer's to appear in their 30s or 40s.
The study comes after several experimental drugs have failed to prevent declines in memory and thinking even though they succeeded in removing amyloid from the brains of patients in the early stages of Alzheimer's. Those failures have eroded support for the idea that amyloid is responsible for a cascade of events that eventually lead to the death of brain cells.
"Many of us think of that as the ultimate test of the amyloid hypothesis," says Dr. Randall Bateman, a professor of neurology at Washington University School of Medicine in St. Louis."If that doesn't work, nothing will work."
The new experiment, called the DIAN-TU primary Prevention Trial, is scheduled to begin enrolling patients by the end of the year.
An explanation with a history
The amyloid hypothesis can be traced to Dr. Alois Alzheimer, a pathologist who first described the disease that would bear his name in 1906.
Alzheimer was working at a psychiatric clinic in Munich, where he had the chance to conduct an autopsy on a woman who died at 50 after experiencing memory loss, disorientation, and hallucinations. He observed that the woman's brain had an "unusual disease of the cerebral cortex," including "senile plaque" usually seen in much older people.
In the 1980s, scientists showed that these plaques were made of beta-amyloid, a substance that exists in many forms in the brain, from single free-floating molecules to large assemblies that form the sticky plaques reported by Alzheimer.
Since that discovery, most efforts to treat Alzheimer's have involved drugs that target various forms of amyloid. And that approach still makes sense, Bateman says.
"We have 30 years of solid data, thousands of studies that all say this is sufficient to cause Alzheimer's," he says.
But doubts about the amyloid hypothesis have been rising as the list of drug failures has grown in the past decade.
For example, Bateman and a team of researchers were unable to halt Alzheimer's in a study of patients who got the anti-amyloid drug gantenerumab.
"What we found was that it had reversed the amyloid plaques in their brains," Bateman says. "We did not have evidence of a thinking-memory benefit."
Even so, Bateman and many other scientists think it's too soon to abandon the amyloid hypothesis.
"Penicillin, a great breakthrough, failed its first two clinical trials," Bateman says. "Fortunately, people didn't say, oh, the antibiotic theory is a bad idea and we should give up on it."
Hints of a benefit
Bateman is encouraged by results from recent studies of anti-amyloid drugs, even the ones that have not prevented cognitive decline.
Gantenerumab, for example, seemed to delay several brain changes associated with the death of brain cells, he says.
And the experimental drug lecanemab did appear to slow down the loss of memory and thinking in a study of nearly 1,800 people with early Alzheimer's disease, according to a statement from the drug's maker.
Many studies of anti-amyloid drugs may have failed because they were given to people who already had amyloid plaques in their brains. At that point, Bateman says, it may not be possible to stop the process that ultimately kills off brain cells.
So Bateman is optimistic about the upcoming prevention trial, which will start treatment much earlier.
"My prediction is it will work, and it will work fantastically," he says. "If we can really prevent the plaques from starting and taking off and those downstream changes from going, my prediction is those people will never get Alzheimer's."
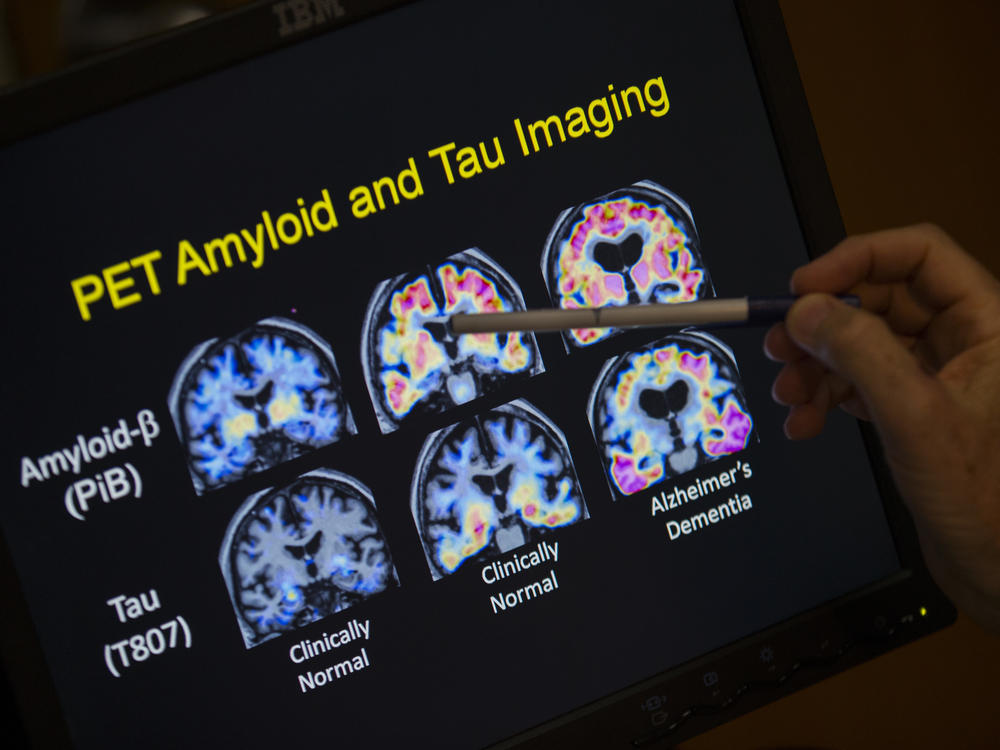
The prevention study is based on the idea that when amyloid begins to build up, it causes a series of changes in the brain, says Dr. Eric McDade, a professor of neurology at Washington University who will oversee the experiment.
These changes include the appearance of toxic tau tangles inside neurons, the loss of connections between neurons, inflammation, and, ultimately, the death of brain cells involved in thinking and memory.
"What we're trying to do is to prevent that amyloid pathology from developing in the first place," McDade says.
That sort of prevention, though, will mean starting treatment long before symptoms appear.
"At the point of somebody having symptoms, we know now that they probably have had amyloid in their brain for one to two decades," McDade says.
So the four-year study will enroll about 160 people from families with dominantly inherited Alzheimer's disease. This form of dementia is caused by rare, inherited gene mutations that cause Alzheimer's to develop in middle age, often in a person's 30s and 40s.
"The earliest they can come in is 25 years before we anticipate they would start to develop symptoms," McDade says. "For most of these families, that actually puts them in their mid 20s when we're going to start this trial."
Like the earlier study that failed, this one will use the anti-amyloid drug gantenerumab.
The short-term goal is to make sure that amyloid plaques do not appear. Then, researchers will look to see whether this prevents the appearance of other markers of Alzheimer's effects on the brain.
One of these markers is the presence of neurofibrillary tangles, a toxic version of a protein called tau that forms disorganized threads inside a neuron. These internal tangles disrupt a cell's ability to transport chemicals and nutrients from place to place and to maintain connections with other cells.
Another marker is brain atrophy, a shrinkage in one or more brain areas caused by the loss of neurons and the connections between them.
"If we prevent amyloid pathology from developing and these other markers continue to develop and unfold," McDade says, "this would be one of the best ways to say, listen, amyloid is really not what we should be targeting."
Copyright 2022 NPR. To see more, visit https://www.npr.org.