Section Branding
Header Content
Augusta University Set To Vaccinate Employees Next Week If Pzifer Wins FDA Approval
Primary Content
Augusta University Health System is making distribution plans for two COVID-19 vaccines ahead of the FDA’s approval for emergency use authorization.
The U.S. Food and Drug Administration on Thursday will consider the COVID-19 vaccine developed by Pfizer.
The United States on Wednesday recorded its highest number of daily deaths — 3,140 — since the pandemic began. In Georgia, 9,073 people have died of COVID-19.
Emergency room visits related to COVID-19 increased to 6% statewide over the two-week period Nov. 21 to Dec. 4, according to the state health department.
Approval for emergency use authorization is expected, and officials with Augusta University have a plan to start vaccinating employees before the end of the month.
A decision about Pfizer's vaccine will be voted on sometime between Friday and Sunday, and then Moderna's EUA panel will convene Thursday, Dec. 17, with a decision made between Dec. 18 and 20.
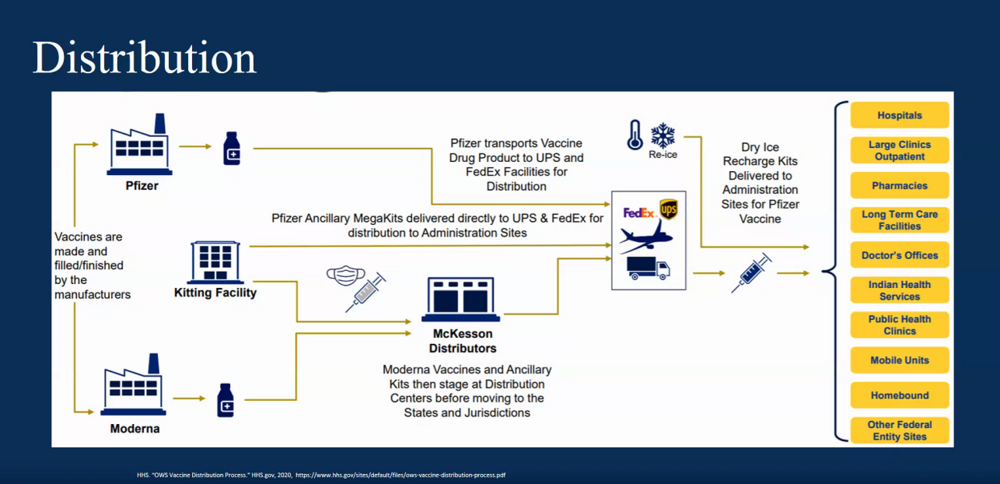
Dr. Joshua Wyche, the director of Pharmacy for Ambulatory Services at Augusta University Health, said 48 hours is a good barometer for successful distribution post EUA decision.
He expects AU to distribute the Pfizer vaccine toward the end of next week and the Moderna vaccine the week of Dec. 21.

"The phased approach is simply because in a perfect world supply and demand would be equivalent, but, unfortunately, in the case of the initial COVID-19 vaccine, demand will extraordinarily be higher than the supply that is available," he said, "even with the unprecedented at-risk manufacturing that has taken place by both Pfizer and Moderna."
MORE: COVID-19 Vaccines' Development An 'Unprecedented Success,' Emory Says
It could take up to 18 months before everyone who wants a vaccine can get one, Wyche said.
Because supply is limited and demand far exceeds what is available, no one will be required to take a COVID-19 vaccine.