Section Branding
Header Content
How Feds Decide On Remdesivir Shipments To States Remains Mysterious
Primary Content
By the second week in July, COVID-19 cases in North Carolina were climbing fast.
With nearly 19,000 diagnoses over the previous two weeks, only five states recorded more new coronavirus cases than North Carolina did.
"Today is our highest day of hospitalizations and our second-highest day of cases," Gov. Roy Cooper, a Democrat, announced on July 9, standing behind a podium in the state's Emergency Operations Center. "Please continue to treat the virus like the deadly threat that it is."
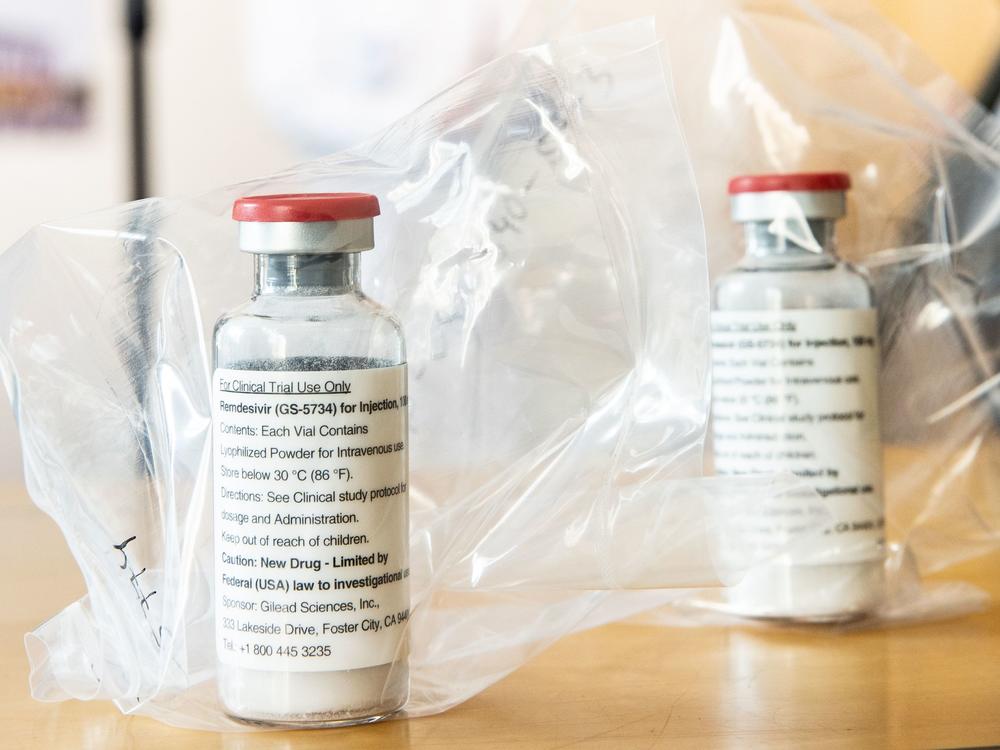
One of the few treatment options for patients seriously ill with COVID-19 is the antiviral drug remdesivir. Authorized by the Food and Drug Administration in May for emergency use in the pandemic, remdesivir is in short supply. The federal government has taken on the responsibility for deciding where vials of the medicine should go.
Between July 6 and July 19, the federal Department of Health and Human Services allocated shipments of remdesivir to 31 states.
North Carolina wasn't one of them.
Loading...
"We then very suddenly heard that we were getting none on the next supply," said Cameron Wolfe, an infectious disease physician at Duke University Medical Center, adding that other hospitals in the state were equally shocked. "We all had supplies that we could see were being used and would basically run out around the same time."
Things appeared to have gotten better since the early days of remdesivir distribution in May when hospitals seemingly got their remdesivir allocation at random. But doctors around the country say they still don't fully understand HHS' system for distributing the drug.
NPR attempted to dig into federal data to understand how the government was making its decisions about remdesivir, but only a few of the data points used in the allocation process are public. Still, NPR has learned that some states, such as North Carolina, appear to have at times been allocated insufficient amounts of remdesivir, while others were offered more than they needed.
Loading...
Each week in July and early August, some states and territories were earmarked less remdesivir than they were offered the week before or none at all, even as their number of patients hospitalized with COVID-19 was trending upward, an NPR analysis found. Meanwhile, some states were allocated enough remdesivir to treat every hospitalized COVID-19 patient more than once that week.
In the first full week of August, HHS allocated enough remdesivir to Vermont for it to treat all of its hospitalized COVID-19 patients nearly six times, according to an analysis of HHS allocation and estimated hospitalization data, using a standard five-day course of the drug.
HHS said it offered Vermont enough remdesivir for 51 patients that week, representing "only a fraction" of its hospitalized COVID-19 patients the previous week, when it made the determination. But according to HHS data, Vermont hasn't had more than 19 estimated patients battling the coronavirus in the hospital on any one day since July 1. When asked about this, an HHS spokesperson explained that there were many more additional "suspected" cases that it took into consideration but they aren't included in the estimates available in the public dataset.
Still, Vermont's open data portal indicates that confirmed cases were in the single digits the week before HHS offered it enough remdesivir to treat 51 people. Even with suspected cases factored in, Vermont's tally of hospitalized COVID-cases didn't go above 20.
Tim Stetson, who leads Vermont's medical logistics team, said he isn't so sure about the HHS spokesperson's tally of remdesivir hospitalizations in his state. "Fifty-one for a single period of time just seems like a really big number," he said. He explained that the state has turned down all HHS allocations of remdesivir since early July because it had plenty of the drug left over from when it received donated cases allocated by HHS in May and June.
Earlier in the pandemic, Gilead pledged to donate its initial supply of 1.5 million remdesivir doses. When the inventory ran out, the company announced a price of $520 per vial at the end of June. Since then, the federal government secured nearly all of Gilead's commercial remdesivir supply through September, and is continuing to determine allocation to states.
In a clinical study, remedesivir shortened hospital stays for seriously ill COVID-19 patients by four days. A panel of experts convened by the National Institutes of Health recommends that limited supplies of remdesivir be prioritized for hospitalized patients with COVID-19 who need supplemental oxygen, not including patients who require "high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation. ..."
Heather Pierce, senior director for science policy and regulatory counsel of the Association of American Medical Colleges, said she thinks HHS is allocating remdesivir "with the best of intentions." But a lack of overall transparency and confusion surrounding how to submit data properly to HHS could cause mismatches between remdesivir allocations and need.
"It's both a relative success story and also a cautionary tale," she said. "By August of 2020, we should be very, very good at this and we're not."
Back in North Carolina, the state health department was baffled.
"We had thought, based on our COVID data, we would receive an allocation, but always knew a zero allocation was a possibility," Amy Ellis, a North Carolina Department of Health and Human Services spokeswoman, told NPR in an email.
State officials acted fast to let hospitals know there was no remdesivir on the way, and that they'd have to rely on what remained of their previously donated remdesivir. When they asked a regional HHS coordinator why they didn't get an allocation for the week, they didn't get an answer. "The state was told that the decisions were coming directly from the White House Task Force and that the question would be passed up to that team," Ellis wrote.
Maybe the data North Carolina entered didn't make it into the new reporting system, they thought. But a meeting in the middle of the month told them their data went into the HHS system just fine, according to Ellis. The state still doesn't know what happened, but it got an allocation the following week.
For its part, an HHS spokesperson told NPR in an email that the agency didn't skip an allocation for North Carolina despite its data showing the state wasn't offered any remdesivir during the first week of commercial allocation in early July. The spokesperson said HHS has no outstanding questions from the state.
Sometimes a skipped allocation makes sense. A state that received excess remdesivir might get skipped in future rounds. Illinois already had so much unused remdesivir from when HHS was allocated donated doses in May and June, that HHS skipped it for allocation three weeks in a row in July, even though the state had more new COVID-19 diagnoses than 38 other states during that time.
"We had several weeks where our case rate was coming down very aggressively, and yet we kept getting large allocations of drugs that we didn't need," said Michael Ison of Northwestern University in Evanston, Ill., an infectious disease physician who is involved in remdesivir research. "And when you're not paying for it, of course, you'll take it, put it in storage and whatnot."
Officials from Illinois did not return NPR's request for comment.
HHS said that its methodology for determining how much remdesivir to allocate to each state changed on July 15. Before then, allocations were based on the proportion of hospitalized COVID-19 patients in a state compared with the rest of the country. Now the determination also includes data on new hospital admissions. These data aren't publicly available, however.
Asked why HHS doesn't make public all data and formulas used to determine allocation, a spokesperson said that "any entity that plays a role in the allocation or distribution of remdesivir," including health departments, can have access to the data. HHS and the assistant secretary for preparedness and response also do weekly calls with state health officials and national health care associations, so they can ask questions.
But that leaves a lot of people out of the loop.
"The information that's being used to make those determinations is not equally accessible to all," said Pierce of the Association of American Medical Colleges. "So while this is the most clarity that we have on any aspect of this pandemic, the bar is not very high on these issues."
Northwestern's Ison said even though Illinois wasn't short of remdesivir, he wonders about the implications of having excess vials of the drug. Does it sit in storage while a hospital elsewhere is running out of its supply for the week, which reportedly happened last month in Florida and Texas?
"I don't know that we can say they're doing a good job," he said. "I don't know that we have the data."
The American Society of Health-System Pharmacists surveyed hospital pharmacists at 112 hospitals in early July and found that a third of them didn't have enough remdesivir to treat all their "eligible" COVID-19 patients. And 3% said they completely exhausted their supply.
Still, HHS seems to be getting better at allocation, said Michael Ganio, senior director of pharmacy practice and quality at the American Society of Health-System Pharmacists. The shift to looking at new COVID-19 hospital admissions, for instance, puts less weight on the number of patients on ventilators for whom remdesivir is now considered less beneficial. He said he hasn't heard complaints about allocation since early May.
But he said the same issues could crop up again.
"I would say keep us on speed dial, because we're going to be repeating this all over again with the vaccine," he said.
You can contact NPR pharmaceuticals correspondent Sydney Lupkin at slupkin@npr.org.
Copyright 2020 NPR. To see more, visit https://www.npr.org.