Caption
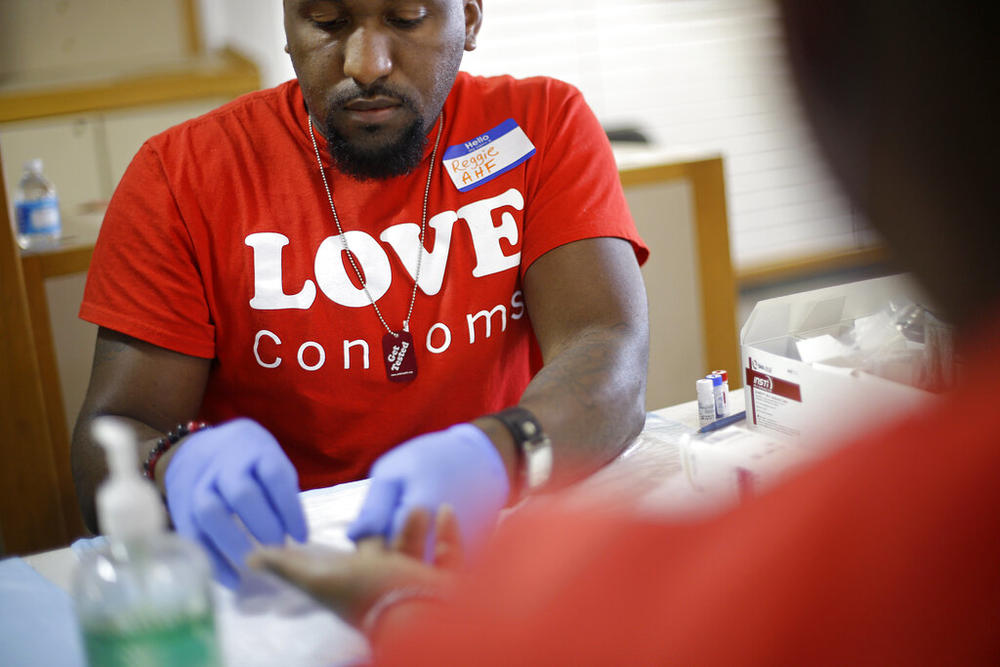
Reggie Batiste (left) program manager with AIDS Healthcare Foundation, administers a free HIV test as part of National HIV Testing Day in 2013 in Atlanta.
Credit: AP Photo/David Goldman
LISTEN: A clinical trial in Georgia aims to address the state’s high rates of HIV. GPB’s Ellen Eldridge reports the PReP drug offers more options for preventing the disease.

Reggie Batiste (left) program manager with AIDS Healthcare Foundation, administers a free HIV test as part of National HIV Testing Day in 2013 in Atlanta.
Three clinical trial sites in Atlanta contributed significantly to the approval for lenacapavir, which is the first and only pre-exposure prophylaxis medication (PrEP) administered once every six months, by enrolling the population at highest risk for HIV acquisition.
Dr. Colleen Kelley is the lead author of the New England Journal of Medicine paper that describes the full results of lenacapivir for PrEP based on the PURPOSE 2 study.
She led clinical trials at Emory's HOPE Clinic, while Dr. Kimberley Workowski ran trials at Emory Midtown and Valeria Cantos Lucio was the principal investigator at the Grady research site.
"We contributed significantly by enrolling the population at highest risk for HIV acquisition in Atlanta, namely, young Black populations, including gay, bisexual, transgender, and gender non-binary people," Cantos Lucio said. "So that was our, you know, first contribution to the approval of lenacapavir for PrEP."
The most recent data shows Georgia has the highest rates of HIV in the country.