Section Branding
Header Content
Beijing Reports Tens Of Thousands Inoculated In 1st Days Of COVID-19 Vaccine Campaign
Primary Content
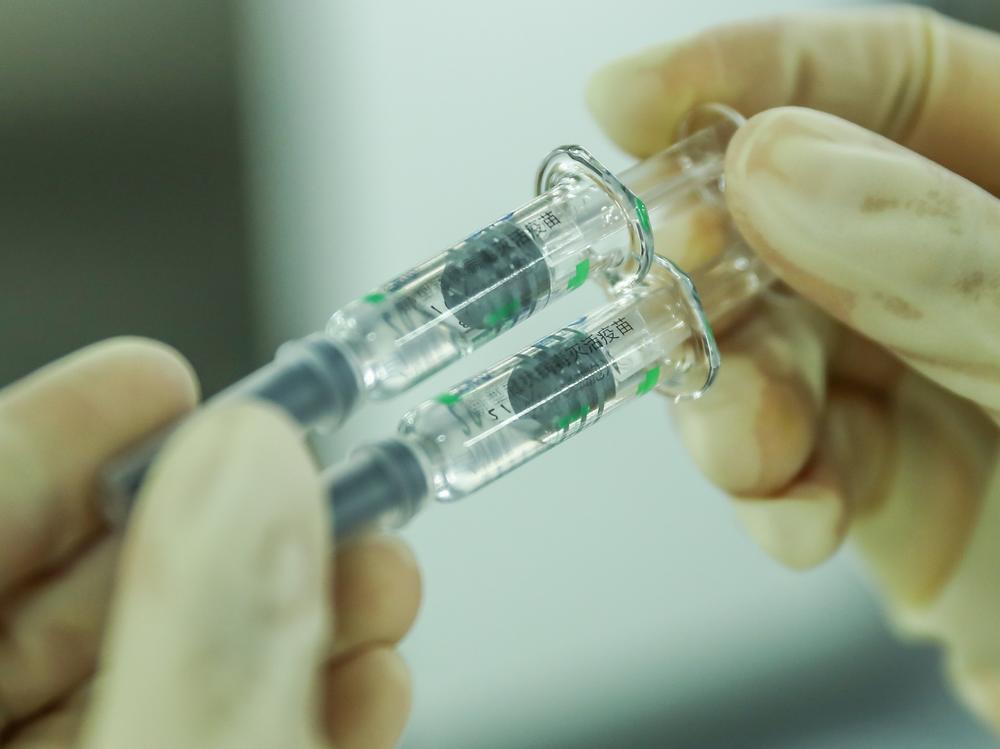
Beijing says it inoculated more than 73,000 people in the first two days after China's first domestic COVID-19 vaccine was approved for commercial use.
China's capital has set up 220 vaccination centers around the city to dole out the two-step vaccine. The elderly and front-line medical workers will receive the first doses.
The shots are made by a subsidiary of Chinese state vaccine-maker Sinopharm, which said on Thursday that its vaccine is 79% effective overall. The company has not yet released more detailed clinical data that might explain why that rate is lower than results from human trials it conducted in the United Arab Emirates, where the vaccine was deemed 86% effective.
However, Chinese state regulators cleared the vaccine for broader public use in China on the same day. The UAE was the first to approve Sinopharm's vaccine for commercial use in early December. Bahrain quickly followed, and a handful of countries have already placed orders for Sinopharm's vaccines, including Pakistan and Ukraine.
Since summer, Sinopharm and another vaccine-maker, Sinovac, have already injected millions of Chinese citizens, many of them state employees, under emergency use guidelines.
Copyright 2021 NPR. To see more, visit https://www.npr.org.