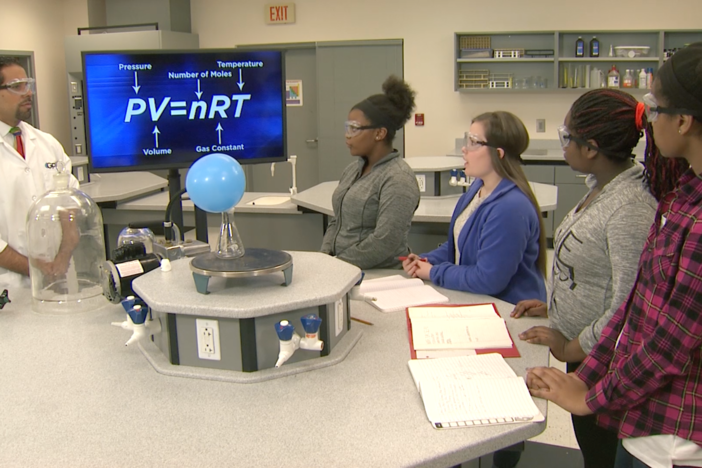
Segment B: Reaction Rate Lab
Students discuss their hypotheses about altering the rates of reaction in a dissolving antacid tablet and conduct their experiments, using variables to determine if their assumptions hold true.
Segment B: Reaction Rate Lab
Students discuss their hypotheses about altering the rates of reaction in a dissolving antacid tablet and conduct their experiments, using variables to determine if their assumptions hold true.
Science
Obtain, evaluate, and communicate information about how to refine the design of a chemical system by applying engineering principles to manipulate the factors that affect a chemical reaction.
Plan and carry out an investigation to provide evidence of the effects of changing concentration, temperature, and pressure on chemical reactions.
Construct an argument using collision theory and transition state theory to explain the role of activation energy in chemical reactions.
Obtain, evaluate, and communicate information about the Kinetic Molecular Theory to model atomic and molecular motion in chemical and physical processes.
Plan and carry out an investigation to calculate the amount of heat absorbed or released by chemical or physical processes.
activation energy - the minimum amount of energy that reactants must possess in order to undergo a specific reaction.
catalyst - a substance that provides a lower energy pathway for reactants to convert to products without getting used up or changing itself.
collision theory - a theory that states that particles must collide in order to react.
effective collision - collisions that result in a successful reaction.
ideal gas - gas that follows the behavior described by kinetic molecular theory.
ineffective collision - collisions that do not result in a successful reaction.
kinetic energy - the energy of motion.
kinetic molecular theory - A theory of the thermodynamic behavior of matter, especially the relationships among pressure, volume, and temperature in gases, based on the dependence of temperature on the kinetic energy of the particles of a substance.
reaction rate - the change in concentration of a reactant or product over time.
The Chemistry Matters teacher toolkit provides instructions and answer keys for labs, experiments, and assignments for all 12 units of study. GPB offers the teacher toolkit at no cost to Georgia educators. Complete and submit this form to request the teacher toolkit. You only need to submit this form one time to get materials for all 12 units of study.