Segment E: Periodic Trends Part II
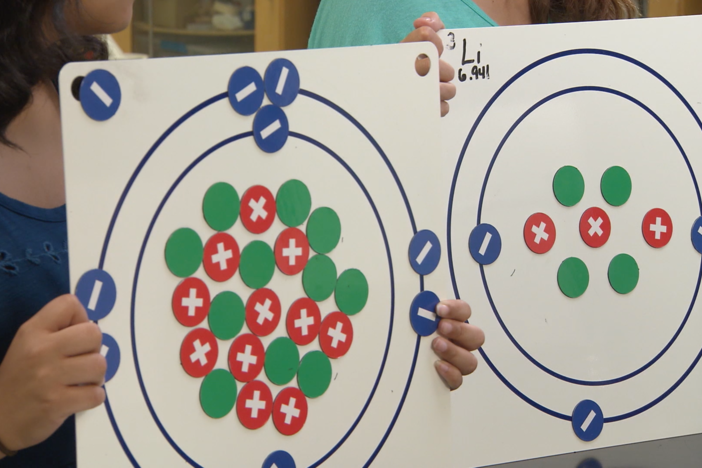
In this segment, the students make predictions about electronegativity and atomic radius across periods and columns.
Segment E: Periodic Trends Part II
In this segment, the students make predictions about electronegativity and atomic radius across periods and columns.
Science
Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms (i.e. including atomic radii, ionization energy, and electronegativity).
Develop and use models, including electron configuration of atoms and ions, to predict an element's chemical properties.
Obtain, evaluate, and communicate information from the Periodic Table to explain the relative properties of elements based on patterns of atomic structure.
Develop and use models to compare and contrast the structure of atoms, ions and isotopes.
Analyze and interpret data to determine trends of the following:
- Number of valence electrons
- Types of ions formed by main group elements
- Location and properties of metals, nonmetals, and metalloids
- Phases at room temperature
Use the Periodic Table as a model to predict the above properties of main group elements.
Obtain, evaluate, and communicate information about the structure and properties of matter.
Develop models (e.g., atomic-level models, including drawings, and computer representations) by analyzing patterns within the periodic table that illustrate the structure, composition, and characteristics of atoms (protons, neutrons, and electrons) and simple molecules.
anion - a negatively charged ion.
atomic number - the number of protons in the nucleus of an atom.
atomic radius - the distance from the atom's nucleus to the outermost energy level.
average atomic mass - a weighted average of all of the isotopes of that element in the universe.
cation - a positively charged ion.
effective nuclear charge (Zsubeff) - the net positive charge experienced by the valence electrons from the nucleus.
electron - a tiny particle with a negative charge that is found outside the nucleus of an atom.
electron configuration - the order in which electrons are arranged in an atom.
electronegativity - the ability of an atom to attract additional electrons.
energy sublevel - a smaller part within a primary energy level.
excited state - an atom, ion or molecule with an electron in a higher than normal energy level than its ground state.
ground state - the lowest energy state within electron orbitals.
Hund's Rule - When placing electrons in equal energy orbitals, electrons should not be paired until each equal energy orbital contains one electron.
ion - an atom with a positive or negative charge.
ionization energy - the amount of energy required to remove one valence electron from an atom.
isotope - the same element with different numbers of neutrons.
model - a physical, conceptual, or mathematical representation of a real phenomenon whose purpose is to explain and predict the observed phenomenon.
orbital - a region of space around the nucleus of an atom where an electron is likely to be found.
Pauli exclusion principle - when an orbital holds two electrons, the electrons much have opposite spin.
quantum - a specific amount of energy that can be absorbed by an electron as it moves from ground state to excited state, or released by an electron as it falls from the excited state back to ground state.
subatomic- any smaller part of an atom such as a proton, neutron, or electron.
valence electrons - electrons on the outer-most energy level of any atom.
valence shell - the outer-most energy level of an electron.
The Chemistry Matters teacher toolkit provides instructions and answer keys for labs, experiments, and assignments for all 12 units of study. GPB offers the teacher toolkit at no cost to Georgia educators. Complete and submit this form to request the teacher toolkit. You only need to submit this form one time to get materials for all 12 units of study.